Kybella
Conveniently located to serve Chicago and North Shore
Get Kybella injections in Chicago as a solution to stubborn fat under your chin. If you have struggled with a double chin and no amount of exercise and dieting has helped, then this may be the ideal time to try Kybella. If surgery sounds too invasive, then it’s time to consider this effective minimally invasive option.
What is Kybella?
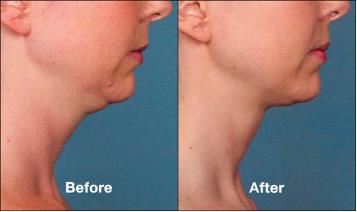
 Many people feel self-conscious about the appearance of a “double chin.” This cosmetic concern can be caused by loose tissue and/or excess fatty tissue in the upper neck, creating a less defined profile. While a double chin has traditionally been corrected with liposuction or a neck lift, MetropolitanMD now offers the non-surgical solution known as KYBELLA™, which treats excess fat through special injectable treatments.
Many people feel self-conscious about the appearance of a “double chin.” This cosmetic concern can be caused by loose tissue and/or excess fatty tissue in the upper neck, creating a less defined profile. While a double chin has traditionally been corrected with liposuction or a neck lift, MetropolitanMD now offers the non-surgical solution known as KYBELLA™, which treats excess fat through special injectable treatments.
This innovative procedure is the first of its kind to be FDA-approved for the reduction of submental fullness (or excess fat under the chin). Its active ingredient–deoxycholic acid, which occurs naturally in the body–breaks down fat when injected into the trouble area under the chin. As this unwanted tissue gradually depletes and is removed via metabolic processes, patients can begin to see a more desirable neck and chin contour.
Is Kybella Right for Me?
If you are over 18 years old and have no allergies to any of the ingredients in Kybella, then you may be eligible for Kybella injections. It is important to have good skin elasticity, since Kybella does not address sagging skin. If you are pregnant, breastfeeding, or obese, you may not a good fit for this procedure.
Your Consultation
A physical examination of your chin and neck will be performed during your consultation. The firmness of your skin will be evaluated, as well as the amount of adipose tissue in the submental region (chin). Your medical history will be reviewed, including your current medications and any underlying illnesses.
A tailored treatment plan will be developed to achieve the best results. To track your progress, photographs may be taken for before-and-after comparison. If you have any questions, feel free to ask and we will be happy to answer them.
What to Expect During Your Kybella Treatment
To begin the KYBELLA™ session, one of our experienced professionals will carefully inject the formula utilizing a very fine needle. For added comfort, patients can choose to have a numbing agent administered prior to KYBELLA™. The entire process is fairly quick, taking approximately 15 to 20 minutes, and is performed in-office.
While a single Kybella treatment can provide noticeable results, multiple treatments are typically required. Most patients require a series of two to four sessions to reach their optimal results. Each treatment is typically scheduled around two or so weeks apart in order to allow the area to heal between treatments. A personalized treatment plan will be created during the consultation following evaluation of the patient’s physical needs and cosmetic goals.
Kybella Recovery
Recovery following KYBELLA™ is relatively short. Minimal downtime is typically needed for most people, though patients may experience slight swelling, bruising, and temporary numbness. However, these side effects should fade over the first few days following each session.
The Cost of Kybella
The cost of your Kybella treatment in Chicago will be based on several factors like the amount of Kybella required and the number of treatments needed to provide the patient with their ideal results. During your consultation, our team can go over what to expect during your Kybella treatment and let you know what the expected cost of your Kybella treatment in Chicago will be. Financing options are also available.
As with any medical or surgical procedure, there are risks involved with a Kybella injection. Please review the Kybella website for additional information. Kybella Website
Dr. Rachel is a superb and skilled surgeon. There are few if any doctors that have the high regard that Dr. Rachel has for his patients. His surgical skills are surpassed by none.
Contact Us Today
You don’t have to let your double chin prevent you from feeling comfortable with the way you look. Contact MetropolitanMD today to schedule a consultation for your Kybella treatment in Chicago. Dr. John D. Rachel is a quadruple board-certified cosmetic surgeon who is committed to providing all of our patients with the outstanding aesthetic care they need.








